EDUCATION
HCOOCH CH2 H2O: Structure, Properties, Applications, and Safety of Hydroxyethyl Formate
Introduction
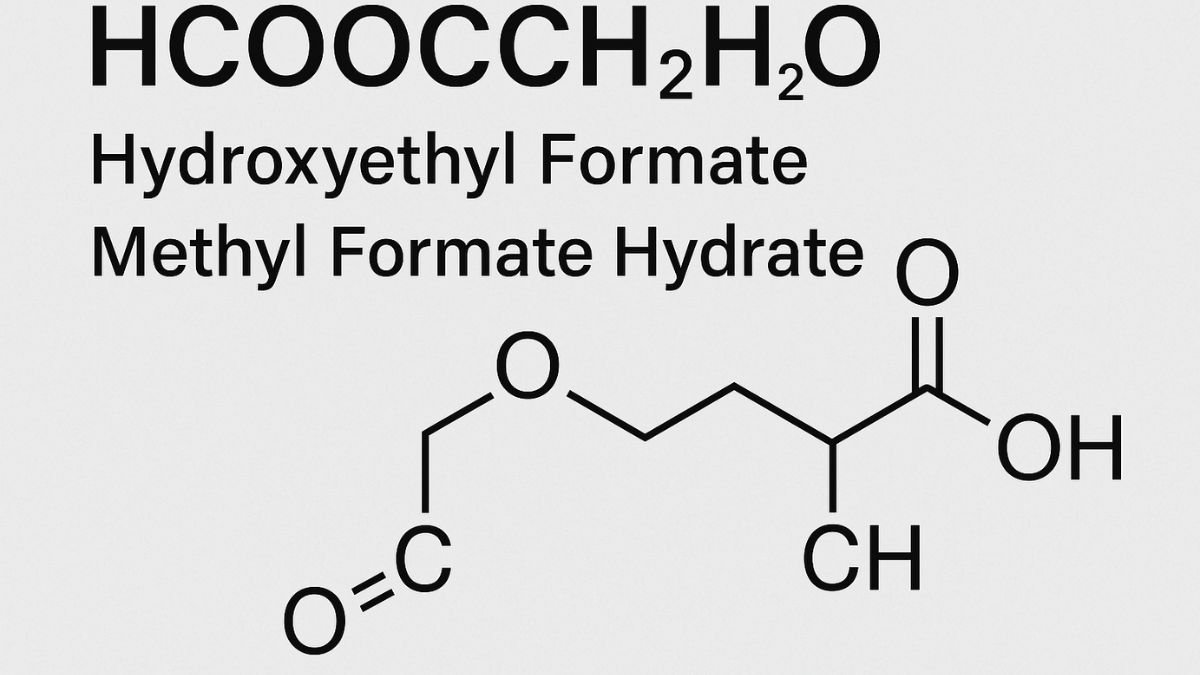
Hydroxyethyl formate, chemically denoted as HCOOCH CH₂ H₂O, is a lesser-known but highly functional organic compound derived from formic acid and ethylene glycol. Often studied under the umbrella of formate esters, this compound plays a critical role in green chemistry and sustainable industrial applications. From pharmaceutical solvents to polymer precursors and even battery electrolyte components, Hydroxyethyl formate is emerging as a valuable asset in modern chemical engineering.
Chemical Structure & Molecular Formula
The compound HCOOCH CH₂ H₂O structurally consists of a formate ester (HCOO–), a methylene group (CH₂), and a water molecule or hydroxyl component (H₂O or OH). The correct IUPAC name is 2-Hydroxyethyl formate. It is essentially the ester formed when formic acid reacts with ethylene glycol.
- Molecular Formula: C₃H₆O₃
- Molar Mass: ~90.08 g/mol
This molecule contains both polar (hydroxyl, ester) and non-polar (methylene) functionalities, making it versatile in solubility and reactivity.
Synthesis of Hydroxyethyl Formate
Hydroxyethyl formate is typically synthesized through an esterification reaction between formic acid and ethylene glycol under controlled conditions:
Formic Acid + Ethylene Glycol → Hydroxyethyl Formate + Water- Reaction Conditions:
- Temperature: 60–80°C
- Catalyst: Acidic (e.g., sulfuric acid or PTSA)
- Time: 2–4 hours for optimal yield
- Industrial Yield: Up to 85% purity after distillation
This synthesis is relatively green, using non-toxic reagents and producing minimal waste.
Physical and Chemical Properties
Hydroxyethyl formate exhibits several unique physical characteristics:
| Property | Value |
|---|---|
| Boiling Point | ~175–180°C |
| Density | ~1.18 g/cm³ |
| Solubility | Miscible in water and alcohols |
| Odor | Sweet, mild ester-like |
| Appearance | Colorless to pale yellow liquid |
It is a polar compound, facilitating interactions with both water and organic solvents.
Applications and Industrial Uses
Hydroxyethyl formate is gaining popularity across multiple domains:
- Green Solvent:
- Environmentally friendly replacement for chlorinated hydrocarbons.
- Used in surface coatings, paints, and adhesives.
- Battery Electrolytes:
- Utilized as a co-solvent in lithium-ion batteries for enhanced stability.
- Pharmaceutical Industry:
- Serves as a mild solvent or intermediate in drug formulation.
- Polymer Industry:
- Acts as a building block in producing polyesters and polyurethanes.
- Cosmetics and Personal Care:
- Humectant and fragrance carrier in creams and lotions.
Safety, Toxicity & Handling Precautions
Though relatively benign, hydroxyethyl formate still demands responsible handling:
- Hazards:
- May irritate skin and eyes upon contact.
- Inhalation of vapors can cause mild respiratory irritation.
- Storage:
- Store in a cool, dry, well-ventilated area.
- Keep away from strong oxidizers and heat sources.
- Handling:
- Use gloves, goggles, and lab coats.
- Ensure proper ventilation in workspaces.
- Disposal:
- Dispose according to local environmental regulations.
FAQs (People Also Ask)
Q1: What is HCOOCH CH₂ H₂O?
A: It is hydroxyethyl formate, an organic ester compound used in solvents, pharmaceuticals, and polymers.
Q2: How is hydroxyethyl formate synthesized?
A: By reacting formic acid with ethylene glycol under heat and acidic conditions.
Q3: What industries use hydroxyethyl formate?
A: Battery manufacturing, pharmaceuticals, green chemistry, and cosmetics.
Q4: Is hydroxyethyl formate hazardous?
A: It is generally safe with mild irritant properties; handle with standard safety gear.
Q5: Can hydroxyethyl formate be used in fuel cells or biodegradable plastics?
A: Research is ongoing; its ester structure makes it a potential candidate.
Conclusion
Hydroxyethyl formate (HCOOCH CH₂ H₂O) exemplifies how organic chemistry continues to deliver versatile, sustainable solutions for industrial and technological advancements. With applications across solvents, batteries, pharmaceuticals, and cosmetics, and a relatively safe profile, it represents a promising compound for the future. Researchers and engineers should continue exploring its potential, especially in green technologies and sustainable production chains.
EDUCATION
Top Passive Income Ideas for Students in 2025

Being a student often means balancing studies, part-time jobs, and financial pressure. But what if you could earn money even while you sleep or study? That’s the power of passive income. It allows you to make money with minimal ongoing effort once the initial work is done.
Here are some of the best passive income ideas for students in 2025 that involve little to no initial investment and can establish long-term financial freedom.
1. Start a YouTube Channel
You don’t require professional equipment or editing knowledge to begin with. Select a niche such as study tips, tech reviews, or personal stories. Once your videos begin attracting views, you can earn money from ads, sponsorships, and affiliate marketing. The more you post regularly, the more passive income you can make in the long run.
2. Sell Digital Products
If you have design skills, writing prowess, or organizational skills, then sell digital products such as:
- Printable planners
- Study guides
- Resume templates
- Social media graphics
Sell your products on platforms such as Etsy, Gumroad, or Payhip. Once created, they can be sold forever.
3. Create an Online Course
If you know a topic (such as coding, writing, or math), build a brief online course on sites like Udemy or Skillshare. Students globally are always looking for useful, beginner-oriented content. Once live, your course can earn you money each month with little maintenance.
4. Affiliate Marketing
Affiliate marketing allows you to gain a commission for advertising other people’s products or services. You can add affiliate links on your blog, YouTube description, or social media. When another user clicks on your link and buys something, you get a percentage of the sale.
Along the way towards creating online revenues, sites such as Linkhouse can assist students in locating opportunities for guest posting, marketing partnerships, and products to build your content-driven revenues. It’s an all-around helpful place to learn from others and advertise your own digital properties as you grow.
5. Invest in Dividend Stocks (With Caution)
If you’ve got some money stashed away and you wish to make it grow, invest in dividend stocks. These are stocks from companies that give you a share of their earnings periodically. Use platforms such as Robinhood or Webull (where available), but take care to research thoroughly before investing.
6. Create a Blog
It’s simple and cheap to start a blog. Blog about what you love, student life, travel, or technology. The more traffic your blog has, the more you can make money passively from display ads, affiliate links, and sponsored content. Although it will take some time to gain an audience, the payoff in the long run is worth it.
7. License Your Photos
If you are a photography enthusiast, post your images on stock photo sites such as Shutterstock, Adobe Stock, or Pexels. With every download of your photo, you will receive a minimal amount of payment. It’s a wonderful method of converting a hobby into a steady income.
Conclusion
Making passive income as a student is not just possible—it’s a good idea. These passive income strategies for students can assist you in becoming financially independent, fund your studies, and even establish a future business. Select one or two approaches that suit your skills and remain consistent. Some small effort today can be rewarding for years ahead.
-

 BLOG7 months ago
BLOG7 months agoDiscovering The Calamariere: A Hidden Gem Of Coastal Cuisine
-
 TECHNOLOGY4 months ago
TECHNOLOGY4 months agoAVtub: The Rise of Avatar-Driven Content in the Digital Age
-

 HEALTH8 months ago
HEALTH8 months agoChildren’s Flonase Sensimist Allergy Relief: Review
-

 BLOG8 months ago
BLOG8 months agoWarmables Keep Your Lunch Warm ~ Lunch Box Kit Review {Back To School Guide}
-

 HEALTH8 months ago
HEALTH8 months agoTurkey Neck Fixes That Don’t Need Surgery
-

 TECHNOLOGY7 months ago
TECHNOLOGY7 months agoHow to Build a Mobile App with Garage2Global: From Idea to Launch in 2025
-

 HEALTH8 months ago
HEALTH8 months agoMasago: The Tiny Sushi Topping with Big Health Benefits
-

 BLOG7 months ago
BLOG7 months agoKeyword Optimization by Garage2Global — The Ultimate 2025 Guide
